Lucy in the Sky with Diamonds
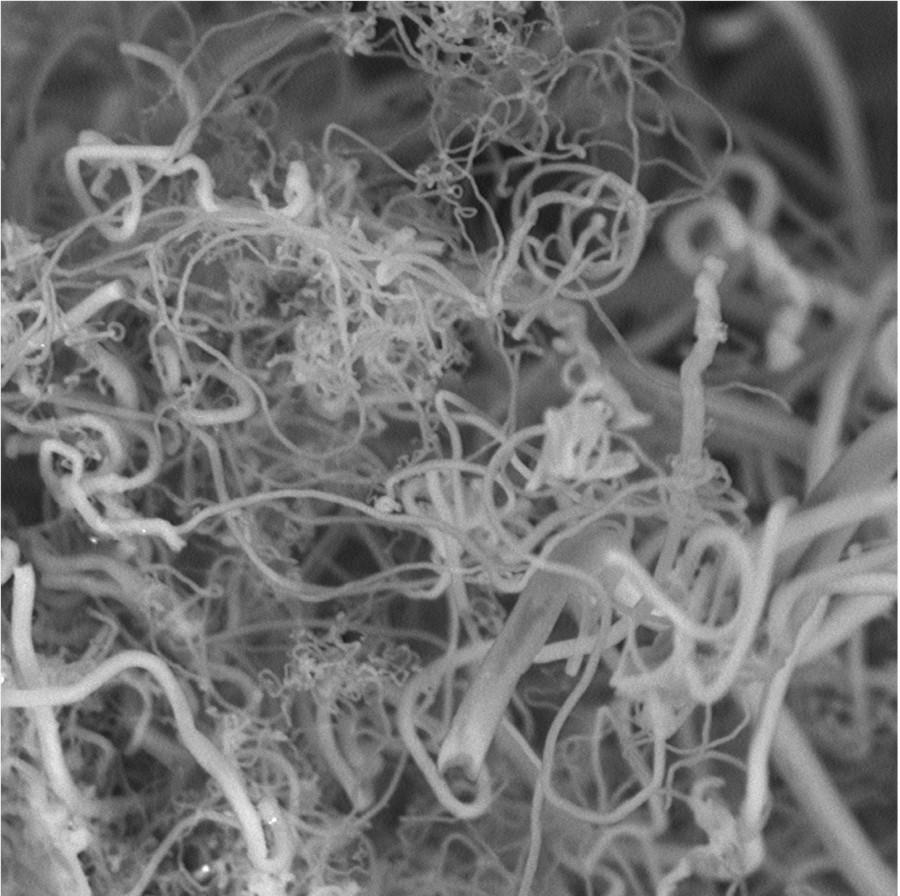
Imagine if a research team discovered a way to completely reverse global warming in 10 years. Pretty cool, right? Except you don’t actually have to imagine it because a team of chemists has found a way to do it. The chemists claim that, given an area less than 10% of the size of the Saharan desert, their process could revert atmospheric conditions to what they were before the industrial revolution within 10 years, an astonishing claim indeed. Stuart Licht, Ph.D., stated that his team has successfully turned atmospheric CO2 into carbon nanofibers — a strong material used in planes, bikes, and all sorts of strong, weight-sensitive objects. He stresses that the process is entirely experimental, and that the largest obstacle ahead of them is to make consistently sized nanofibers, as well as scale up production – but that there’s much potential in this discovery.
The process (affectionately dubbed “Diamonds from the sky”,) involves using solar panels to heat electrolytic cells, causing CO2 to break down in an immersion of molten carbonates at 750 C. Atmospheric air is added to a cell, which dissolves the CO2 when both heat and current are applied through nickel and steel electrodes. Once finished, the nanofibers can simply be collected from the steel electrodes. Powering the electrolysis is achieved through a hybrid solar energy system, which focuses the sun’s ray on a photovoltaic panel to create electricity, and on a second system to create heat which raises the temperature of the electrolytic cell. Such a system runs up a bill of about $1,000 per ton of nanofiber, hundreds of times cheaper than what a ton of nanofiber sells for. Currently, creating nanofiber is an expensive and involved process that generates a large amount of pollution, something this process has the ability to eliminate completely. Although the profit margins and environmental benefits are there, currently the team can only create several grams of nanofibers per hour.
Even in small quantities, however, nanofibers are extremely useful. Potential applications of the material include creating carbon composites for construction, tougher bulletproof vests, sport gear, diapers, filtration systems, batteries, contraceptive devices, wound dressing, tissue engineering, and drug delivery systems. Licht’s team presented this and their other findings at the American Chemical Association, a video of which can be found here. ACS is the world’s largest scientific society, and their last event hosted over 9,000 presentations on a variety of topics. They’re also a nonprofit organization chartered by Congress, with over 150,000 members, and are a world leader in providing chemistry-related research.

Hi! You must be really bored if you're reading this, but here we go. My name is Sebastian Lloret and I'm a Senior at Air Academy this year. I speak English,...