Drug Trial Gone Wrong
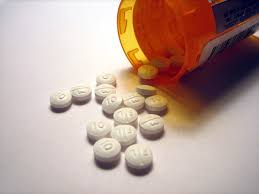
Photo Via Wikimedia Commons under the Creative Commons license – https://en.wikipedia.org/wiki/Drugs_in_the_Australian_Football_League
Every year, thousands of volunteers, often students looking to make extra money, take part in clinical drug trials. Mishaps are relatively rare, but after taking part in a drug trial for Portuguese pharmaceutical firm, Bial, a clinic in north-west France left one person brain dead and five others seriously ill. The drug was designed to treat mood disorders, such as anxiety. The trial was suspended and all others who were taking part in the trial of the orally administered medicine were recalled. In total, CBS Radio News correspondent Elaine Cobbe says six of the 128 people taking part in the trial have been hospitalized. The drug has been administered to ninety people. The volunteers were between the ages of 30 and 50 and most were from the Brittany region of western France, where the lab is located. As paid volunteers, they spent over a week at the approved clinic where they tested the drug by regularly taking increased doses by mouth. The incident is the worst of its kind ever to have taken place in France.
French prosecutors have opened an investigation into the incident, looking at possible charges of “involuntary harm over a period of three months.” They are also trying to determine whether the tragedy was caused by an error in the trial’s procedures or in the substance tested. Mr. Portela said Bial staff in France and Portugal were “working tirelessly to understand the causes of this accident.” Pierre-Gilles Edan, head of the neurology department at the Rennes hospital, said that three of the men were suffering a “handicap that could be irreversible” and another also had neurological problems.
“On my behalf and the behalf of Bial, I would like to express my deepest apologies to the family of the volunteer who died after participating in the Phase I trial of our experimental molecule,” he said. While it was speculated in French media that the drug contained cannabis, the Health Ministry has denied the claims. As mentioned before, such major incidents are rare during the development of a drug, which begins in the laboratory before being animal tested and then goes through three phases of human trials before it can be brought to market.
Medical trials has these three phases to assess a new drug or device for safety and effectiveness. Phase I entails a small group of volunteers and focuses only on safety. Phase II and Phase III are progressively larger trials to assess the drug’s effectiveness, although safety remains paramount. The study in this case was in Phase I of the clinical trial, in which the healthy volunteers took the medication to “evaluate the safety of its use, tolerance, and the pharmacological profile of the molecule,” the minister added in a statement. Again, such major incidents are rare during the development of a drug, so it was shocking for it to occur on this scale.
Another recent example concerns six men in London who were treated for organ failure in 2006 after taking part in a clinical trial for a drug developed to fight auto-immune disease and leukemia. The men now apparently have a higher risk of cancer and autoimmune diseases tied to their exposure to the experimental drug. What can be gathered from these two instances is that all clinical trials have some risk to them, so when you’re looking to make some extra money, be wary of the effects a trial might have.

Citation:
Christensen, Jen. “French Drug Trial: 1 Person Dead, 5 More Hospitalized.” CNN.com. Cable News Network, 8 Jan. 2016. Web. 11 Feb. 2016.

Hey! My name is Lilliana and I am a junior at Air Academy High School. I am the Senior Sports Editor and this is my second year writing for the Jetstream...










Brady Becco • Feb 17, 2016 at 10:12 am
That would be awful to have this happen. Great article!